Scientists from Hokkaido University have developed a fast, environment friendly protocol for cross-coupling reactions, vastly increasing the pool of chemical compounds that can be utilized for the synthesis of helpful organic compounds.
One of the compounds synthesized in this research, 3u, is strongly photoluminescent in resolution (Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021).
Chemical reactions are an important course of in the synthesis of merchandise for a range of functions. For probably the most half, these reactions are carried out in the liquid part, by dissolving the reactants in a solvent. However, there are a big variety of chemical compounds which might be partially or fully insoluble, and thus haven’t been used for synthesis. The beginning supplies required for the synthesis of many cutting-edge organic materials-such as organic semiconductors and luminescent materials-are usually poorly soluble, resulting in issues in solution-based synthesis. Therefore, the event of a solvent-independent artificial strategy to beat these long-standing solubility issues in organic synthesis is very desired to synthesize new priceless organic molecules.
In latest years, artificial methods utilizing ball milling have been used to hold out solvent-free reactions in the strong part. It has been proposed that using ball milling would probably overcome the aforementioned solubility issues in artificial chemistry, however a scientific research for such objective has by no means been carried out.
A group of 4 scientists from Hokkaido University’s Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), led by Associate Professor Koji Kubota and Professor Hajime Ito, have developed a fast, environment friendly, solvent-free protocol for Suzuki−Miyaura cross-coupling response of insoluble aryl halides. The protocol was revealed in the Journal of the American Chemical Society.
Aryl halides are common beginning supplies for the synthesis of organic useful molecules, primarily by the palladium-catalyzed Suzuki-Miyaura cross-coupling response – for which Hokkaido University’s Professor Emeritus Akira Suzuki shared the 2010 Nobel Prize in Chemistry. Although the cross-coupling reactions have been employed for the synthesis of a variety of priceless molecules, insoluble aryl halides usually are not appropriate substrates as a result of Suzuki-Miyaura cross-coupling reactions have primarily been carried out in resolution.
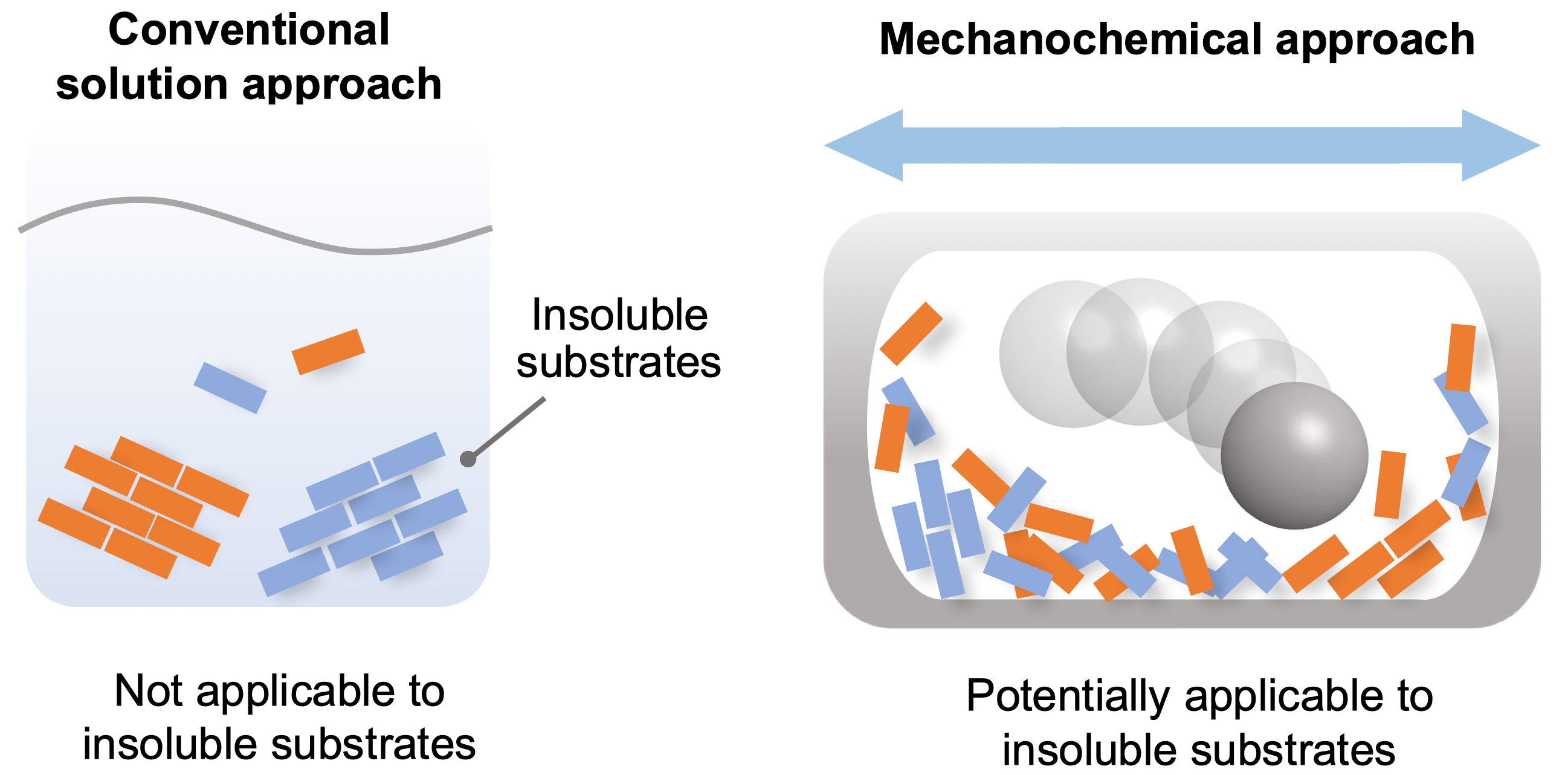
Insoluble reactants are hardly reactive in resolution, however might react in solvent-free techniques utilizing ball milling to drive chemical reactions in the strong state (Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021).
Given this limitation, the scientists targeted on the event of an environment friendly solid-state Suzuki-Miyaura cross-coupling of a lot of extraordinarily unreactive insoluble aryl halides. The key gear consisted of a ball mill, for mixing the reactants; a warmth gun, to extend the temperature at which the reactions happened; and using a catalytic system composed of palladium acetate (the catalyst), SPhos (a high-performance ligand for Suzuki−Miyaura cross-coupling reactions) and 1,5-cyclooctadiene (dispersant and stabilizer).
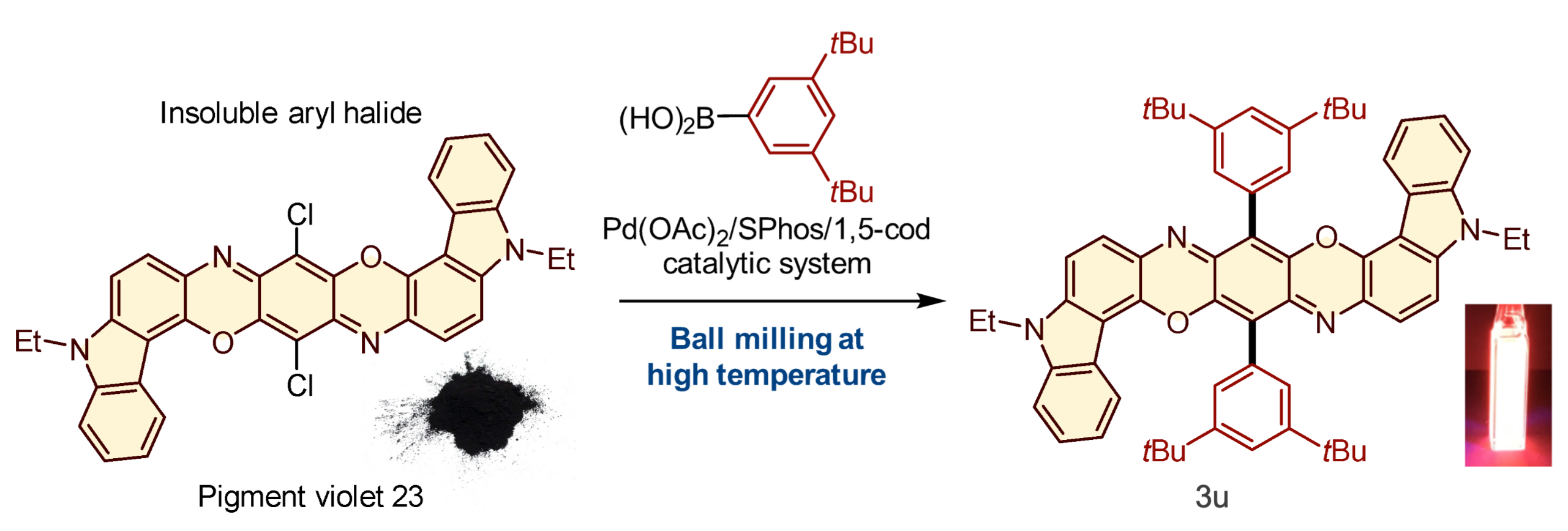
When pigment violet 23, one of many mostly-insoluble aryl halides, was subjected to the response with an aryl boron nucleophile at 120 °C in a ball mill in the presence of the palladium-based catalytic system, it was transformed to a product 3u that was strongly photoluminescent when dissolved in dichloromethane (Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021).
The capstone of this research was the appliance of the solvent-free solid-state response to mostly-insoluble aryl halides. These reactants didn’t yield any merchandise in typical solution-based reactions. The solid-state reactions utilizing the high-temperature ball milling, nonetheless, gave the specified merchandise. Importantly, the group found a brand new robust photoluminescence materials ready from insoluble pigment violet 23.
“The high-temperature ball-milling approach and our catalytic system are important for these cross-coupling reactions of insoluble aryl halides, and the protocol we’ve developed expands the range of organic molecules derived from insoluble beginning supplies,” says Koji Kubota.

Hajime Ito (left), Tamae Seo (heart) and Koji Kubota (proper), authors of this research (Photo: Koji Kubota).
Original Article:
Tamae Seo, et al. Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides. Journal of the American Chemical Society. March 30, 2021.
DOI: 10.1021/jacs.1c00906
Funding:
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grants (18H03907, 17H06370, 19K15547, 20H04795), the Japan Science and Technology Agency (JST) by way of a Core Research for Evolutional Science and Technology (CREST) grant (JPMJCR19R1), and the Institute for Chemical Reaction Design and Discovery (ICReDD), Hokkaido University, established by the World Premier International Research Initiative (WPI), Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.